The key to centromere’s eternal life unravelled
5 Sept 2024
Scientists have solved a decade long question about the mechanism that preserves the centromere, the hub that ensures DNA divides correctly during cell division.
5 Sept 2024
Scientists have solved a decade long question about the mechanism that preserves the centromere, the hub that ensures DNA divides correctly during cell division.

Prof. Jeyaprakash Arulanandam | © LMU
The centromere is a special region of DNA where the cell division machinery attaches to segregate identical copies of the cell's genetic material into newly formed cells. Researchers at LMU and the University of Edinburgh, led by Professor Jeyaprakash Arulanandam (also known as “JP”), have now shown that a protein, known as PLK1, triggers a process that coordinates key proteins at the right place and time during cell division – ensuring each new cell has a centromere in the right location. This finding, reported by the scientists in the journal Science, sheds light on one of life’s most fundamental processes.
“In the human body, around two trillion cells divide every day. Accurate chromosome segregation is the basis for life itself and mistakes can be catastrophic. If centromeres are missing or in the wrong place, then the genetic information is not shared correctly between the dividing cells. In adults, this can lead to many diseases including cancers, whilst in the earliest stages of life it can cause birth defects,” says Arulanandam.
The cell division machinery recognizes centromeres by the presence of multiple copies of a protein known as CENP-A. But every time the cell divides the stocks of this protein at centromeres must be replenished. Over the years, the precise molecular events that allow the centromere to maintain its identity and location through vast numbers of cell divisions have been the focus of intense research.
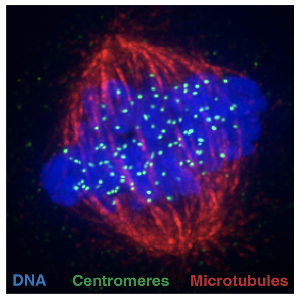
Cell division machinery, made up of microtubule filaments, attaching to centromeres to segregate identical copies of the DNA during cell division. | © Alba Abad Fernandez
Research by another group previously revealed that PLK1 is one of the molecular ‘master’ switches which controls when CENP-A replenishment occurs, but its mechanism of action remained a mystery. In their new study, the researchers used biophysical, biochemical, structural and cell biology techniques to better understand PLK-1 actions.
Their results showed that PLK1 interacts with the so-called Mis18 complex by recognizing a chemical modification, known as phosphorylation, of the proteins Mis18α and Mis18BP1. Previous research, including work by Arulanandam’s team, had revealed that the Mis18 protein complex plays a vital role in replenishing CENP-A levels. The interaction with PLK1 triggers further phosphorylations, which activate the complex.
“We found that PLK1-mediated activation of the Mis18 complex facilitates Mis18 complex binding to and phosphorylation of another protein, known as HJURP, which is responsible for loading CENP-A onto the centromeres”, says Paula Sotelo-Parrilla, researcher in Arulanandam’s Munich team and one of the lead authors of the paper. Together, these changes allow the Mis18 complex to act as a guide, controlling when HJURP binds to the centromere and ensuring that CENP-A is loaded at the right place and time during cell division.
“PLK1 kickstarts a molecular process similar to a relay race that determines how and when key proteins interact. It ensures that CENP-A levels are restored after each round of cell division, preserving the centromere’s integrity”, explains Pragya Parashara, another of the lead authors. “This is one of the cell’s most crucial safeguards and is vital to the correct transfer of genetic material through countless generations of cells – which is essential to the creation and maintenance of life.”
P. Parashara et al.: PLK1-Mediated Phosphorylation Cascade Activates Mis18 Complex to Ensure Centromere Inheritance. Science 2024